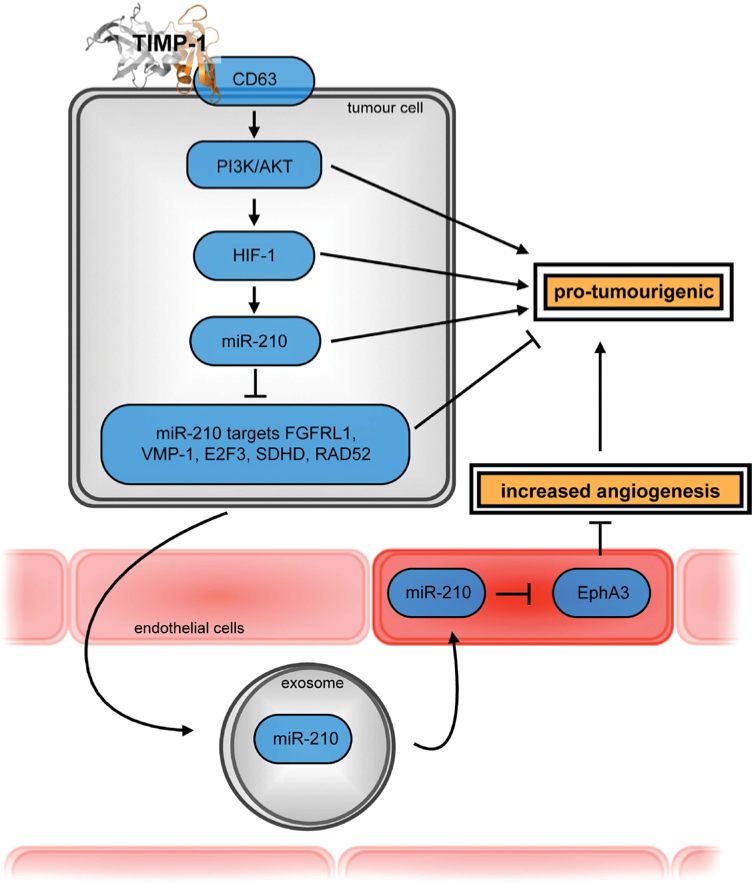
Tumor-associated change of the ‘glycome’ of proteins was recently appreciated as crucial ‘Enabling Characteristic’ for the establishment of all hallmarks of cancer. Species- and tumor cell-specificity of the glycome is rather rarely considered in experimental set-ups in mice or in vitro, when recombinant proteins with undefined glycosylation pattern are employed in functional assays. Tissue inhibitor of metalloproteinases-1 (TIMP-1), a secreted multi-functional factor playing important roles in numerous cancer-associated patho-physiological processes, is a glycoprotein displaying specific N-glycosylation patterns under physiological conditions. Glycosylation-modified TIMP-1 from cancer patients exhibits reduced anti-proteolytic activity, thereby mediating pro-tumorigenic effects. In preliminary experiments, we could show that also the non-canonical tetraspanin (CD63)-mediated signaling function of human TIMP-1 is influenced by glycosylation. In the current project, we aim to unravel the impact of glycosylation on the multi-functionality of human TIMP-1.
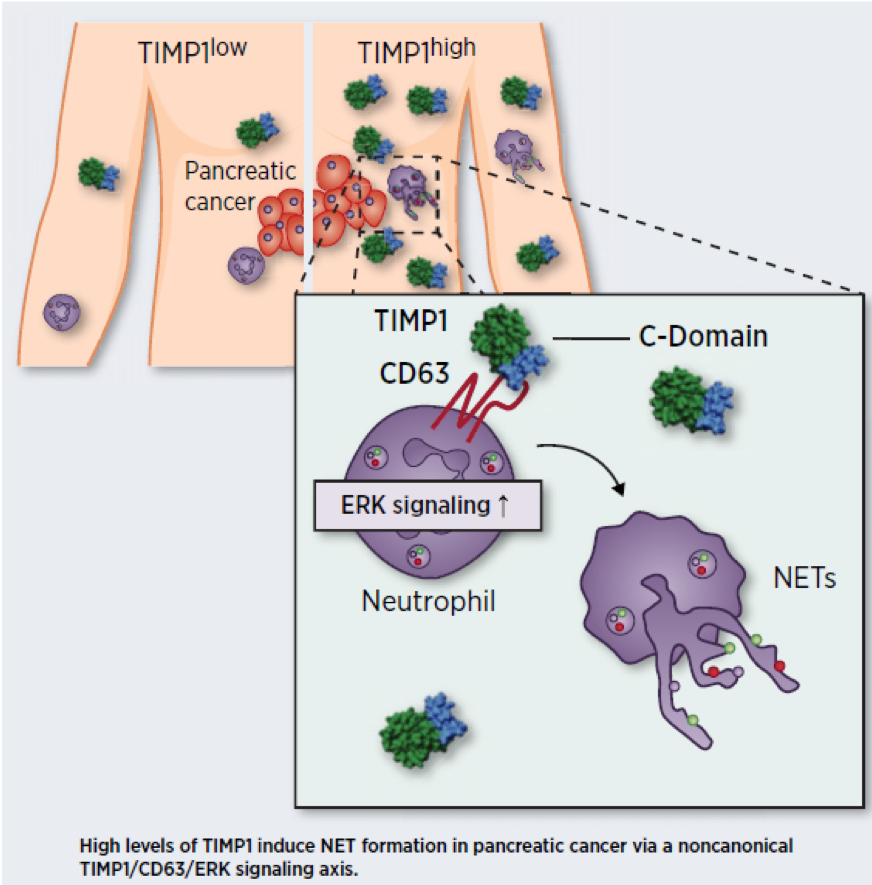
Our previous work showed that Tissue inhibitor of metalloproteinase-1 (TIMP-1) is a protein secreted by most tumors, is found in the blood at levels correlating with progression of liver metastasis in men as well as in cachexia, changes the liver's homeostasis, and changes the metabolic programming of different cell types through its increasingly recognized cytokine and pro-inflammatory function as a receptor ligand. The aim of this research project is to systematically analyze in mouse models and in vitro whether TIMP-1 is a governing factor for the regulation of pro-cachectic cellular metabolic responses via the liver as a central organ regulating metabolism.
Our previous work showed that Tissue inhibitor of metalloproteinase-1 (TIMP-1) is a protein secreted by most tumors, is found in the blood at levels correlating with progression of liver metastasis in men as well as in cachexia, changes the liver's homeostasis, and changes the metabolic programming of different cell types through its increasingly recognized cytokine and pro-inflammatory function as a receptor ligand. The aim of this research project is to systematically analyze in mouse models and in vitro whether TIMP-1 is a governing factor for the regulation of pro-cachectic cellular metabolic responses via the liver as a central organ regulating metabolism.
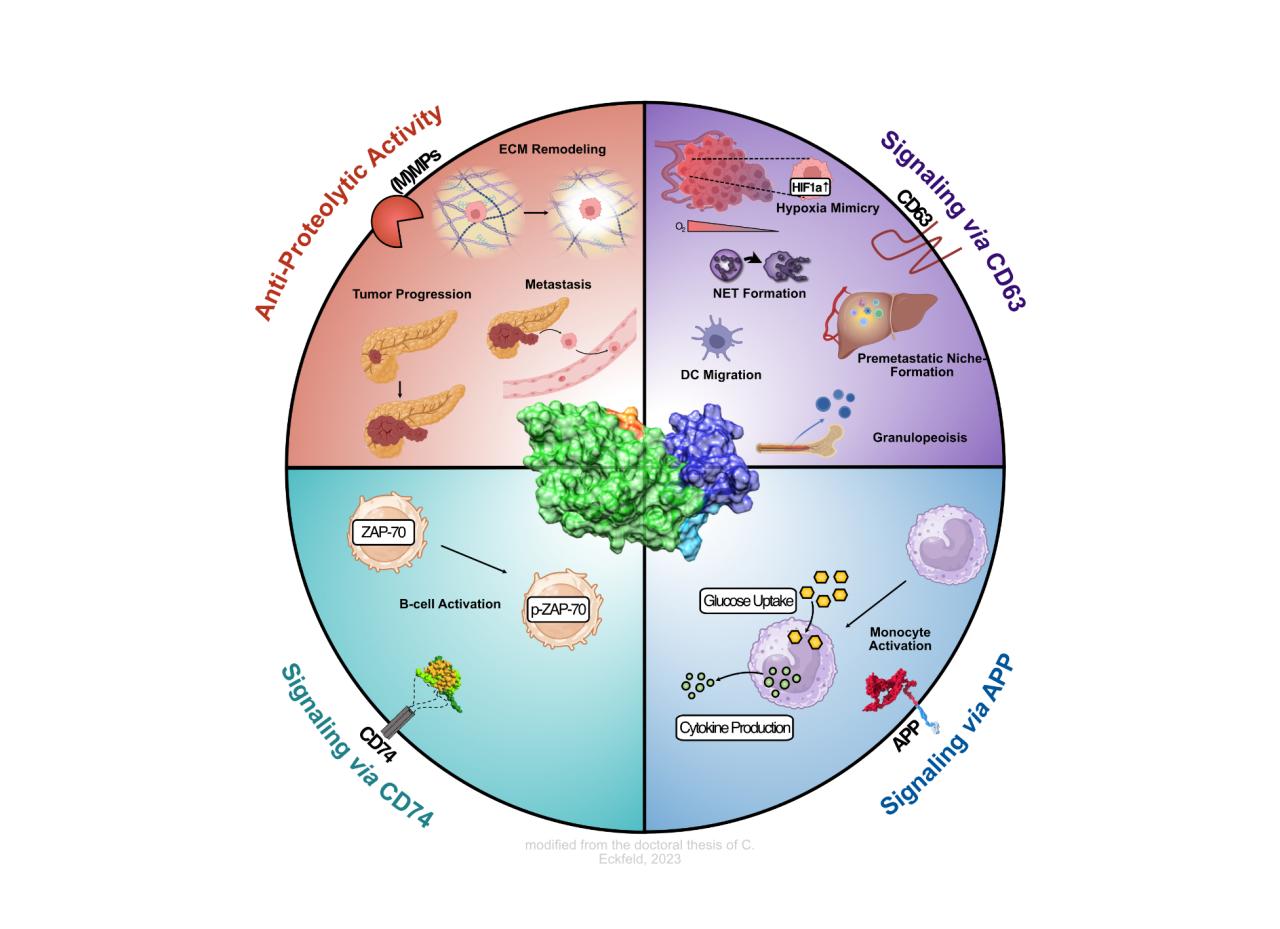
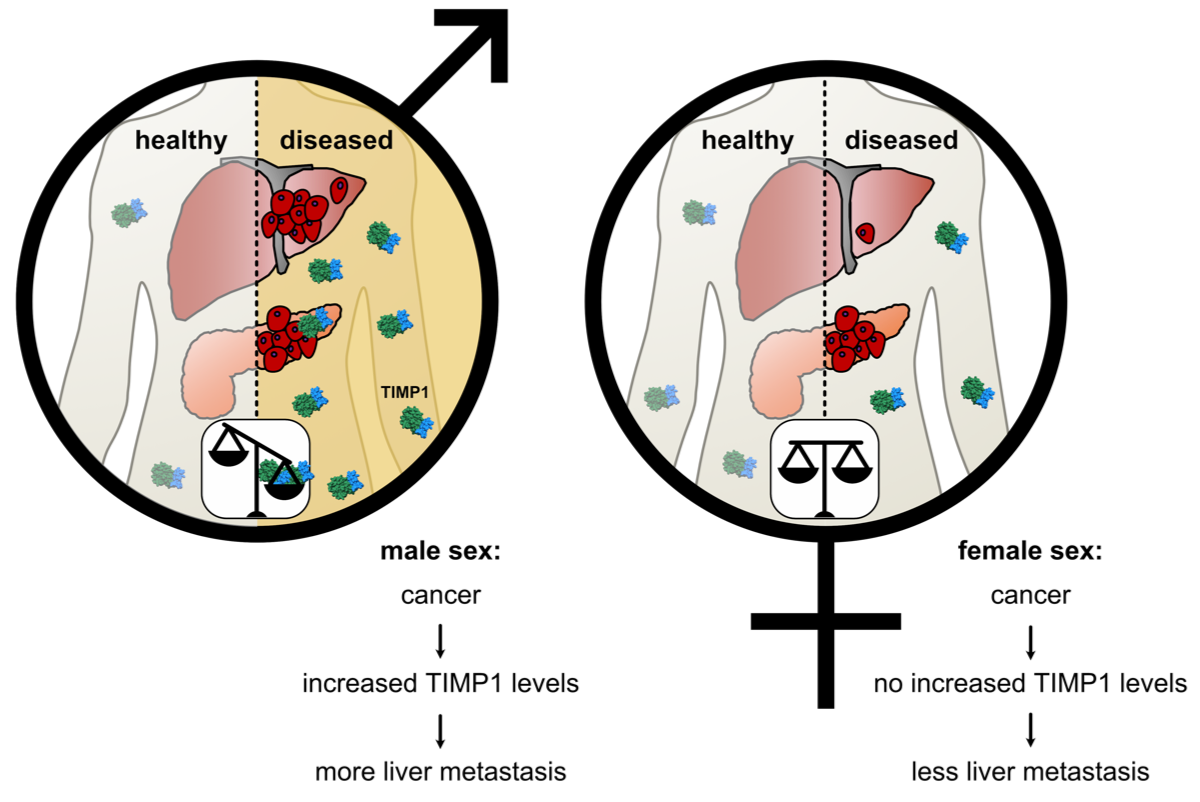
The exceptionally high risk to die from pancreatic cancer decisively arises from its efficient metastasis formation to the liver. Recently we found that this risk is even higher in men than in women and that the tumor-derived secreted factor TIMP-1 (Tissue Inhibitor of Metalloproteinases-1) is responsible for the sex difference in liver metastasis in this cancer entity in a life style-independent manner. In pancreatic cancer, TIMP-1 expression is male-specifically increased, while the levels remain constant in women. The mechanism regulating this sex-specificity of expression of the X-chromosomally located TIMP-1 gene is completely unknown. In this project we follow initial data showing that TIMP-1 is increasingly expressed already at early stages of the progression of this disease by malignant tumor cells and their progenitor cells, respectively, and that epigenetic reprogramming of these cells thereby seems to play a central regulatory role. In fact, based on recent studies, epigenetic reprogramming was classified as novel central ‘enabling characteristic’ during initiation of pancreatic cancer. However, possible sex differences so far have not been considered in those studies. We will investigate the molecular-mechanistic basis for sex-specific increase of TIMP-1 expression.
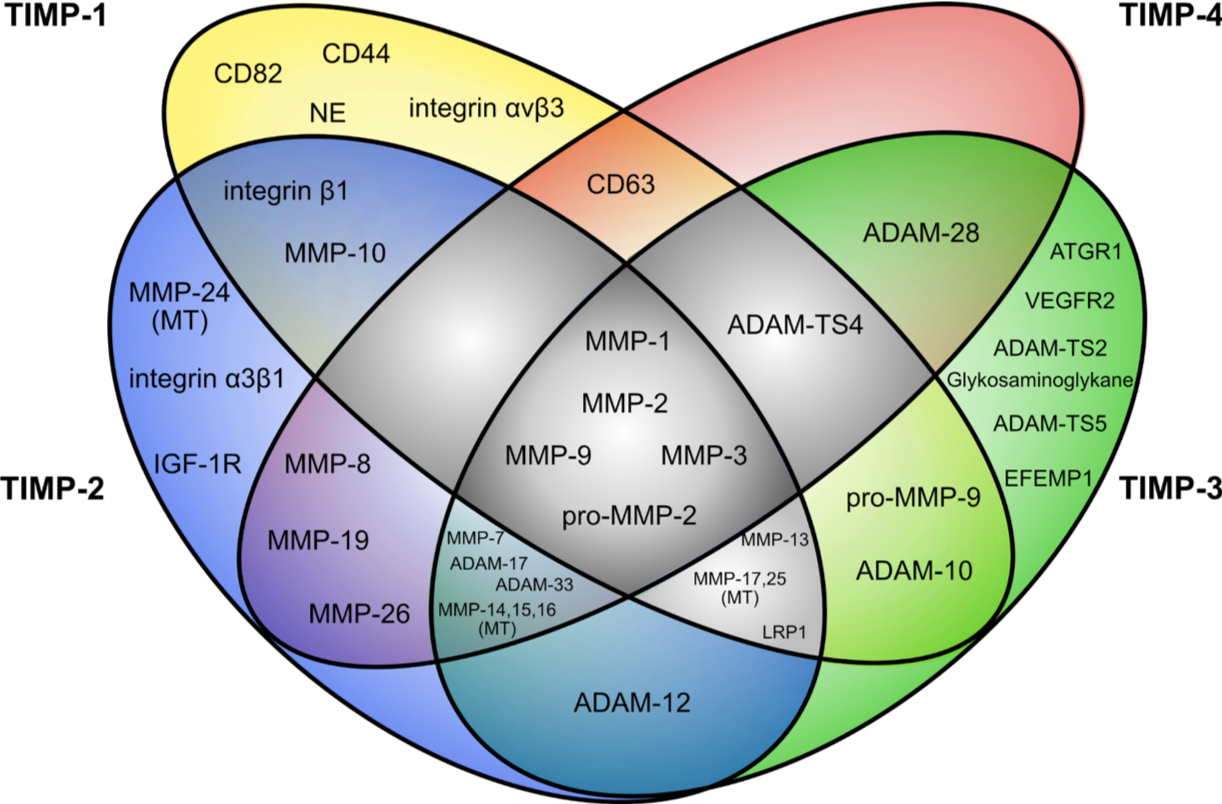
We employ a wide spectrum of technologies encompassing all aspects of molecular cloning/genetic engineering, expression-vector design, biochemistry, cell culture including functional cell-assays, metabolic assays, protein-design and purification, flow cytometry, histology, in silico-modeling (Docking using HADDOCK or Cluspro), statistical analyses etc. in order to explore new structure-function relationships between TIMPs and their receptors, which we also identify in the process.
Through cooperation with colleagues in the clinic we constantly validate our data with material from the clinic towards a transfer of new knowledge from bench to the bedside.
Each of our projects are currently funded by grants of the DFG
